If You Take Away One Thing
Delayed gut signals re-activate the same central amygdala neurons that first encoded a new taste, and PKA-to-CREB signaling primes those neurons for plasticity.
Why Post-Ingestive Feedback Matters
In nature, animals learn what to approach and what to avoid through positive and negative feedback loops. In the context of eating, your body recognizes the flavor upon eating, but the ramifications that it has on your body are felt hours later. The brain must still bind the two. This research paper outlines how a gut signal can reach the central amygdala, light up the original flavor ensemble, and convert a brief event into a lasting memory.
Conditioned flavor aversion (CFA) shows the problem clearly. An animal tastes a flavor and, after a delay, experiences malaise. The behavior is well known. The mouse in the future will lean away from anything resembling this malaise. The open question is how a delayed body signal can target the specific flavor representation and stabilize it. Two parts of the system point to an answer: calcitonin gene-related peptide (CGRP) neurons that carry postingestive malaise from the hindbrain, and the central amygdala, which holds and retrieves flavor ensembles.
The Experimental Setup

The experiment was set up as follows. Mice drank grape Kool-Aid under two conditions. In one, the flavor was new. In the other, it was familiar after four days of safe exposure. Thirty minutes later, mice received a lithium chloride injection to induce gastrointestinal distress. Both smell and taste were available, as in ordinary feeding. The delay between flavor and consequence created a real credit-assignment problem for the brain.
Three Stages of Learning
- Consumption
New flavors activated a central amygdala network. Familiar flavors shifted activity toward lateral septum circuits. Novelty was labeled on the first sip.
- Malaise
During malaise, activity tilted back toward the amygdala network that had encoded the new flavor. The delayed signal did not sweep across the brain uniformly. It re-engaged the same ensemble that was active during consumption.
- Retrieval
On later re-exposure, retrieval again favored the novelty-linked amygdala group. The circuit used at first taste returned at recall. Storage and retrieval were neighbors in the same tissue.
CGRP Neurons Supply the Delayed Signal
Lithium chloride activated CGRP neurons in the hindbrain. Tracing showed projections to the amygdala. Optogenetic activation of CGRP neurons, or of their terminals in the amygdala, reproduced the behavioral and brain-wide effects of lithium chloride. Mice formed stronger aversions to novel tastes. Activity patterns matched the pharmacologic state. Disrupting this input prevented amygdala reactivation during malaise and blocked learning. In short, CGRP neurons were both necessary and sufficient to carry the gut’s verdict to the amygdala.
From Reactivation to Plasticity
If learning depends on CGRP-driven reactivation, the flavor ensemble should change. Using the same readouts before conditioning and at retrieval, the authors found variation among central amygdala neurons. Some cells showed weak reactivation from CGRP input. Others were strongly reactivated. Those strongly reactivated cells later responded more during memory retrieval. Reactivation magnitude predicted retrieval strength, joining the late signal to durable changerather than a transient state shift.
How Familiarization Shifts the Code
Repeated safe exposure strengthens a preference for water and dampens central amygdala activity for the flavor. By selectively reactivating the original ensemble, it maintains or strengthens the representation. The brain separates routine from significance.
The Biochemical Pivot in the Amygdala
Novel tastes increased protein kinase A activity in the central amygdala. Protein Kindse A (PKA) can phosphorylate CREB, which then drives gene programs that stabilize long-term memory. Novelty labels newly active neurons through PKA-to-CREB signaling and prepares them for modification. When the late CGRP signal arrives, those tagged neurons undergo synaptic changes and join the long-term ensemble.
Summary
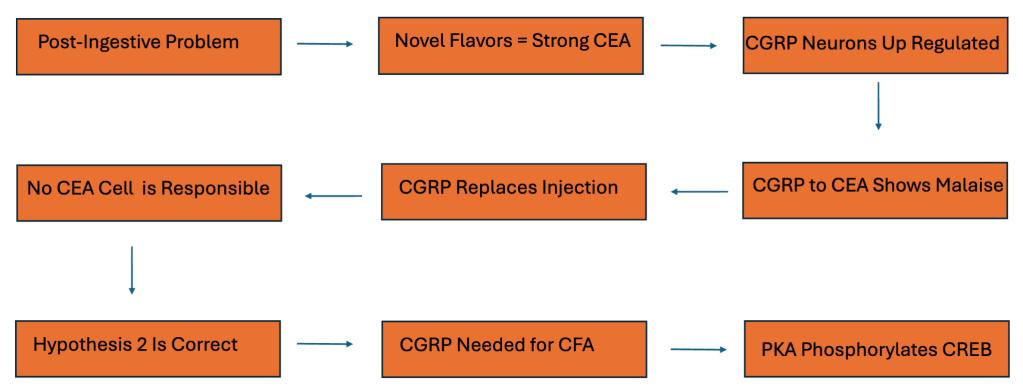
A post-ingestive problem follows a novel flavor, which builds a strong representation in the central amygdala. CGRP neurons then rise in activity, and delivering CGRP input to the CEA produces a malaise signal that can stand in for the usual LiCl injection. Because CGRP can substitute for the injection, no single CEA cell group alone accounts for the effect. Taken together, these results support Hypothesis 2: CGRP signaling is required for conditioned flavor aversion, with PKA phosphorylating CREB to prime CEA neurons for plasticity and long-term memory.
Kenneth’s Opinion
I presented this paper at a Mount Sinai journal club. It was the first time biochemistry and neuroscience truly connected for me. Seeing a gut signal re-engage amygdala neurons, then link to PKA and CREB, showed how molecules and circuits cooperate to form memory. That experience did more than explain a consequence. It opened a path I want to follow, where careful chemistry and careful behavior can be studied together.
FAQ
1) Is this only about aversion, or could it also explain positive learning?
The experiments used malaise, so the focus is aversion. The logic may extend to positive outcomes. If a delayed body signal improves state, a related reactivation rule could strengthen preference, likely through distinct pathways and modulators.
2) Why is the central amygdala central to the story?
It contains ensembles for threat and avoidance. New tastes recruit a central amygdala ensemble at intake. Later, CGRP input re-activates the same ensemble during malaise and at recall. This match across phases marks the central amygdala as the storage and readout site
3) What do CGRP neurons contribute in practice?
They carry the delayed postingestive signal. Turning them on optogenetically mimics lithium chloride and produces aversion. Disconnecting them prevents learning. Their message is both adequate and required to tag the amygdala ensemble with the meal’s consequence.
4) Where do PKA and CREB fit?
Novel flavors raise PKA activity in the amygdala. PKA can activate CREB, which launches gene programs for lasting memory. This creates an eligibility trace. Novelty marks the neurons first. The later CGRP signal drives the synaptic changes that make the memory endure.

Leave a comment