If You Take Away One Thing
Scientists built ion channels that open only when cocaine is present. Turning these channels on in the lateral habenula cut cocaine’s dopamine spike and reduced drug seeking in rats while leaving everyday rewards (ie: food and sex) intact.
Why This Paper Matters
Here is the study I am writing about: a 2025 Nature article by Juan L. Gomez and colleagues that engineered cocaine-activated ion channels to curb cocaine reinforcement in rats; the senior author is Scott M. Sternson, and the paper was published on 27 August 2025. You can read the full article here
Most addiction tools act on dopamine in a broad way and often dull normal motivation. Here the drug serves as its own trigger. When cocaine reaches the brain, it opens a designed channel in a defined circuit that applies a brake at the right time. The work is careful, and aims to stop the negative effects caused by widespread dopamine suppression. Figure 1a sketches this “synthetic physiology” loop, with cocaine activating a receptor that pushes against its own reinforcing effect.

What The Team Built
The group reworked ligand-gated ion channels so that cocaine becomes the key that opens them. They started from an α7 nicotinic acetylcholine receptor binding pocket and grafted it onto cation (5HT3) and anion (GlyR) pores. The result is a pair of cocaine-gated switches for excitation or inhibition. In Fig. 1d–g, you can see how successive mutations raise cocaine potency while dropping acetylcholine sensitivity, leading to the final excitatory channel, coca-5HT3, with low-micromolar cocaine activation and weak responses to endogenous agonists.
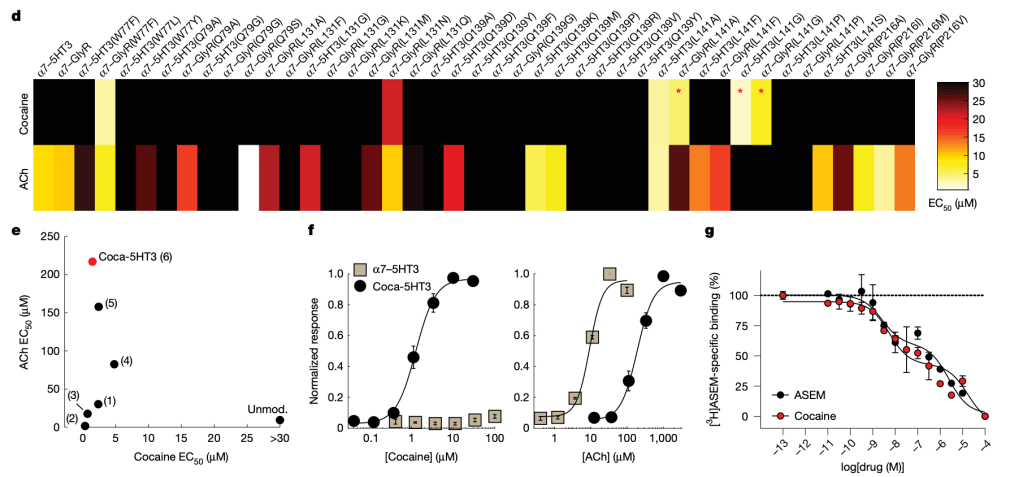
Function checks in neurons confirmed the essentials. Cocaine opened coca-5HT3, depolarized hippocampal neurons, and lowered the current needed to trigger action potentials. The inhibitory partner, coca-GlyR, produced strong cocaine-evoked currents and increased the current required for spiking. Baseline membrane properties were unchanged across groups. Selectivity assays showed minimal activation by choline, cocaine metabolites, and several other amine-containing drugs at physiological ranges, and nicotine potency was markedly lower than in the unmodified receptor.
Where They Put It and Why
They expressed the excitatory coca-5HT3 in the lateral habenula. This is a part of the brain that is highly involved with dopamine systems. Cocaine normally suppresses lateral habenula activity for a short window, which favors intake. Giving these neurons a cocaine sensor flips that response so that cocaine now excites an anti-reward node.
What Happened in Behavior
Rats pressed a lever for intravenous cocaine across doses. With coca-5HT3 in the lateral habenula, the dose to response curve shifted downward, so animals pressed less and consumed less per unit doses that usually seen with self-administration. Training performance on food was similar across groups, which helps separate the cocaine effect from general performance. Control behaviors such as sucrose preference, operant sucrose responding, and open-field locomotion were unchanged. See Fig. 3e-h.

What Happened to Dopamine
Two independent readouts told the same story. PET imaging with the dopamine-sensitive tracer [18F]fallypride showed smaller cocaine-induced displacement in ventral striatum after coca-5HT3 was expressed in the lateral habenula. Fibre photometry with a GRABDA sensor in nucleus accumbens also recorded blunted dopamine rises across doses.
Why This Is Notable
Timing is the key advantage. The channel opens only during cocaine exposure, which avoids the broad effects of constant inhibition. The approach also works as a platform. Similar receptors could be engineered for other drugs to test circuit questions with better precision. Selectivity screens and intact natural-reward controls indicate that the effect is tied to cocaine reinforcement rather than a general loss of motivation.
Kenneth’s Opinion
This hits the blend I like: precise chemistry tied to a single circuit and a clear behavioral readout. Cocaine becomes a timed key, the lateral habenula answers, and you can watch both behavior and dopamine change in step. I like the match between the PET maps and the GRABDA traces. I also like that food-related measures stay stable. If this level of control holds when adapted to other substances, the field gets a cleaner way to test causal ideas without flattening normal drive.
FAQ
1) Does this shut down normal rewards like food or water?
No. Sucrose preference, operant responding for sucrose, and open-field activity were unchanged without cocaine (Extended Data Fig. 6).
2) Why target the lateral habenula instead of dopamine cells directly?
The lateral habenula sits upstream and naturally restrains dopamine systems. Activating it only when cocaine is present lets the brain apply its own brake at the right moment (Fig. 3a–d).
3) How selective are these channels for cocaine?
They were tuned to respond to cocaine at brain-relevant concentrations and showed minimal responses to choline, cocaine metabolites, and several related drugs in the tested ranges (Extended Data Fig. 2–3).
4) What proves dopamine was reduced?
Two measures. PET with [18F]fallypride showed smaller displacement in ventral striatum, and GRABDA photometry in nucleus accumbens showed blunted rises during cocaine exposure (Fig. 4a–h).
5) Is this a therapy?
Not yet. This is a research tool in animals. Viral delivery, long-term safety, and human specificity would need careful work before any clinical step.
Leave a comment